Alcohols Phenols and Ethers-Test Papers
CBSE Test Paper-01
Class - 12 Chemistry (Alcohols, Phenols and Ethers)
- Alcoholic compounds react
- Only as nucleophiles.
- both as nucleophiles and electrophiles.
- only as electrophiles
- None of these
- The increasing order of acidic strength of the following is:
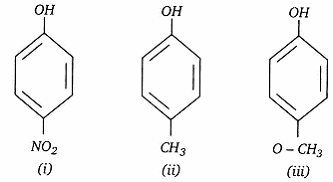
- (ii) < (iii) < (i)
- (iii) < (ii) < (i)
- (i) < (ii) < (iii)
- (i) < (iii) < (ii)
- Dow's process involves
- Nucleophilic substitution
- Electrophilic addition
- Nucleophilic addition
- Electrophilic substitution
- Aldehydes are reduced to the corresponding alcohols by addition of hydrogen in the presence of catalysts to form
- None of these
- tertiary alcohols
- primary alcohols
- secondary alcohols
- What is the correct order of reactivity of alcohols in the following reaction?
- 3° > 1° > 2°
- 1° > 2° > 3°
- 1° < 2° > 3°
- 3° > 2° > 1°
- Write IUPAC names of the compounds.
- Write IUPAC names of :-
- Write IUPAC name of
- What is Picric acid? How is it prepared from phenol?
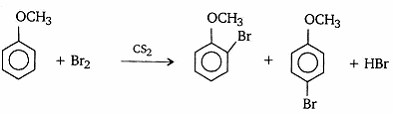
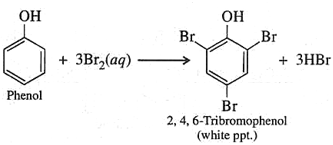
- Give the structural formula and name of the product of following reaction: phenol is treated with excess of aqueous bromine.
- Write the chemical reaction in formation of l - propanol to 2 - bromo propane.
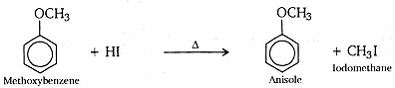
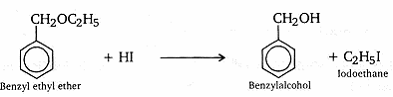
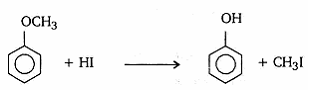
- Write the equation of the reaction of hydrogen iodide with
- 1-Propoxypropane
- methoxybenzene
- benzylethyl ether
- Write the structure of the major products expected from the following reactions:
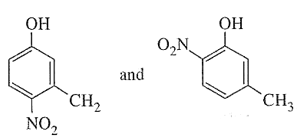
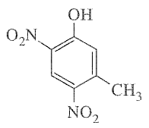
- Mononitration of 3-methylphenoI
- Dinitration of 3-methylphenol
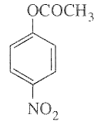
- Mononitration of phenyl methanoate
- During preparation of ester from alcohol and acid, water has to be removed as soon as it is formed?
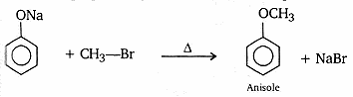
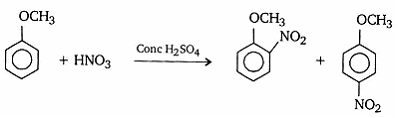
- How is anisole prepared? What happens when it is treated with
- HI
- Nitrating mixture
- Br2 dissolved in CS2?
CBSE Test Paper-01
Class - 12 Chemistry (Alcohols, Phenols and Ethers)
Solutions
- (a) Only as nucleophiles.
Explanation: The functional group of the alcohols is the hydroxyl group, –OH. Unlike the alkyl halides, this group has two reactive covalent bonds, the C–O bond and the O–H bond. The electronegativity of oxygen is substantially greater than that of carbon and hydrogen. Consequently, the covalent bonds of this functional group are polarized so that oxygen is electron rich and both carbon and hydrogen are electrophilic. - (b) (iii) < (ii) < (i)
Explanation: The nitro-group is an electron-withdrawing group. The presence of this group in the para position decreases the electron density on the benzene ring. which in turn decreases the electron density on the oxygen of O-H bond. As a result, it is easier to lose a proton. Also, the p-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid. On the other hand, methoxy group is an electron-releasing group. Thus, it increases the electron density on the oxygen of the O-H bond and hence, the proton cannot be given out easily. For this reason, para-nitrophenol is more acidic than para-methoxyphenol. - (a) Nucleophilic substitution
Explanation: The Dow process is the electrolytic method of bromine extraction from brine, and was Herbert Henry Dow's second revolutionary process for generating bromine commercially.
Dow's Process may also refer to the hydrolysis of chlorobenzene in the preparation of phenol. Benzene can be easily converted to chlorobenzene by electrophilic aromatic substitution. It is treated with dilute sodium hydroxide at 350 °C and 300 bar to convert it to sodium phenoxide, which yields phenol upon acidification. This reaction is quickened manifold in the presence of electron withdrawing groups (such as -NO2) ortho and/or para to the halogen group. - (c) primary alcohols
Explanation: RCHO + H2 RCH2OH- Aldehydes and ketones are most readily reduced with hydride reagents.
- The reducing agents LiAlH4 and NaBH4 act as a source of 4 x H- (hydride ion)
- Overall 2 H atoms are added across the C=O to give H-C-O-H
- Hydride reacts with the carbonyl group, C=O, in aldehydes or ketones to give alcohols.
- The substituents on the carbonyl dictate the nature of the product alcohol.
- Reduction of methanal (formaldehyde) gives methanol.
- Reduction of other aldehydes gives primary alcohols.
- Reduction of ketones gives secondary alcohols.
- (d) 3° > 2° > 1°
Explanation: The mixture of HCl and ZnCl2 is called the Lucas Reagent. Secondary and tertiary alcohols react via the SN1 mechanism with the Lucas reagent. The ZnCl2 coordinates to the hydroxyl oxygen and this generates a far superior leaving group.
When alcohols react with a hydrogen halide, a substitution occurs, producing an alkyl halide and water:
Scope of Reaction: The order of reactivity of alcohols is 3° > 2° > 1° - 2, 2, 4-Trimethylpentane-3-ol
- 2,3 - Dimethylbutan - 2, 3 -diol
- 1-chloropropane - 2 - ol
- Picric acid is 2,4,6 – trinitrophenol
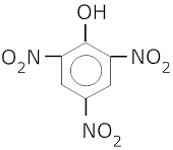
It is prepared from phenol by nitration with conc. HNO3.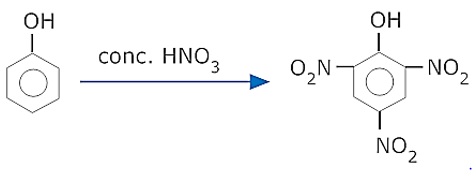
The combined influence of -OH and -CH3 groups determine the position of the incoming group. Keeping in view that both -OH and -CH3 are o- and p-directing groups, the following products are obtained:
The reaction between alcohol and carboxylic acid is reversible and goes in backward direction if water is not removed as soon as it is formed. ROH + RCOOH RCOOR' + H2O
Anisole is prepared by reaction of sodium phenoxide with methylbromide.