Amines-Test Papers
CBSE Test Paper - 01
Class - 12 Chemistry (Amines)
- In a reaction, a secondary amine forms Nitrosoamine (R2N-N=O), which on heating with phenol and conc H2SO4 gives a green colour solution which turns blue on adding alkali. The reaction is called:
- Fries reaction
- Perkin's reaction
- Libermann's nitroso reaction
- Etard's reaction
- If the starting amide has got four carbon atoms and the amine that is formed has got only 3 carbon atoms, then the reaction is called
- Gabriel synthesis
- carbylamines reaction
- Hoffmann bromamide reaction
- Clemmensen reduction
- When one of the following reacts with NaOH, the product is sodium benzoate?
- benzene hydroxide
- benzoic acid
- benzaldehyde
- benzene
- The nitrogen's lone pair in pyrrolidine is best described as occupying what type of orbital?
- s
- sp2
- sp3
- sp
- Which of the following reacts with NaNO2 + HCI to give alcohol?
- C6H5CH2NHCH3
- CH3NH2
- C6H5NH2
- (CH3)3N
- Write IUPAC name of the following compound and classify it into primary secondary and tertiary amine.
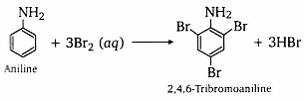
CH3(CH2)2NH2 Write the reaction taking place when aniline reacts with bromine water.
How is orange I prepared?
It is difficult to prepare pure amines by ammonolysis of alkyl halides. Give reasons.
Why are amines more basic than the comparable alcohols.
- Write the chemical equations to illustrate the following reactions:
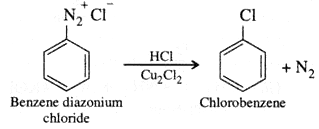
- Sandmeyer reaction.
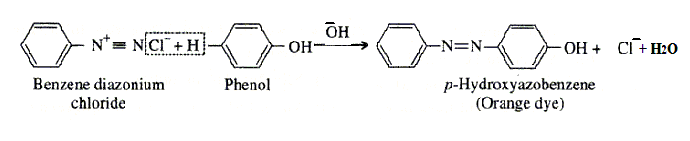
- Coupling reaction.
Describe the method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reaction involved.
- Identify the compounds A, B, C in the following equation.
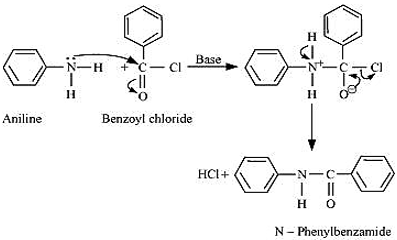
Write an equation of the reaction of aniline with benzoyl chloride and write the name of the product obtained.
- Write short notes on the following:
- Carbylamine reaction
- Diazotisation
- Hofmann's bromamide reaction
- Coupling reaction
- Ammonolysis
CBSE Test Paper - 01
Class - 12 Chemistry (Amines)
Solutions
- (c) Libermann's nitroso reaction
Explanation: This is Libermann's nitroso reaction. Secondary amines react with HNO2 to give N-N nitrosodialkylamine (R2N-N=O). Nitrosamines are water-soluble yellow oils which when warmed with phenol and conc. H2SO4 give green color solution and with alkali, they give a blue color solution. Tertiary amines do not react with nitrous acid. - (c) Hoffmann bromamide reaction
Explanation: In Hoffmann bromamide degradation reaction, the amine formed has one carbon less than the amide.
RCONH2 + Br2 +4NaOH RNH2 + Na2CO3 + 2NaBr + 2H2O - (b) benzoic acid
Explanation: Benzoic acid reacts with NaOH to form sodium benzoate, this is a neutralisation reaction where acid reacts with a base to give salt and water.
C6H5COOH + NaOH C6H5COO-Na+ + H2O - (c) sp3
Explanation: Pyrrolidine is tetrahydropyrrole.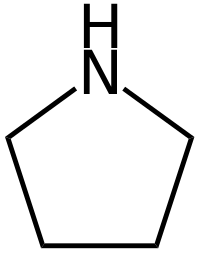
The nitrogen atom in pyrollidine is sp3 hybridized. Two sp3 hybridized orbitals are involved in pairing with carbon, one sp3 hybridized orbital is involved in pairing with hydrogen and one sp3 hybridized orbital is occupied by a lone pair. - (b) CH3NH2
Explanation: Aliphatic primary amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) to form aliphatic diazonium salts, which is unstable and decomposes to give a carbocation and evolve N2 gas. The carbocation so formed reacts with water from medium to give further produce alcohol. Propane-1-amine (1°),
It is obtained by coupling reaction between diazotized sulphanilic acid and alkaline solution of Napthol.
- The process of ammonolysis yields a mixture of primary, secondary, tertiary amines and quaternary salts. This is because the primary amine formed can further react with the alkyl halide to form the secondary amine, which in turn will again react with the alkyl halide to form the tertiary amine, which also reacts with the alkyl halide leading to the formation of quaternary salt. Thus such a reaction would form a mixture of all the four compounds and it will be difficult to get the pure amine.
Amines are more basic than alcohols because lone pair in nitrogen is more available for incoming accepter as compared to oxygen as oxygen is more electronegative than nitrogen.
- Sandmeyer reaction:
- Coupling reaction:
- Sandmeyer reaction:
Primary, secondary and tertiary amines can be identified by Hinsberg's reagent (Benzenesulphonyl chloride C6H5SO2Cl). Primary amines react with Hinsberg's reagent to form sulphonamides soluble in alkali whereas secondary amines form sulphonamides insoluble in alkali. Tertiary amines do not react with Hinsberg's reagent.
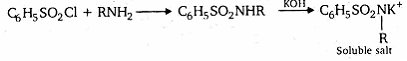
- Carbylamine reaction: Carbylamine reaction is used as a test for the identification of primary amines. When aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide, carbylamines (or isocyanides) are formed. These carbylamines have very unpleasant odours. Secondary and tertiary amines do not respond to this test. For example,
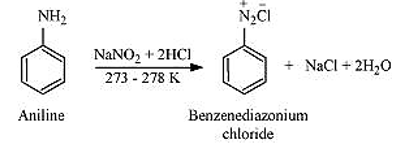
- Diazotisation: Aromatic primary amines react with nitrous acid (prepared in situ from NaNO2 and HCl) at 273 - 278 K to form diazonium salts. This conversion of aromatic primary amines into diazonium salts is known as diazotization. For example,
- Hoffmann bromamide reaction: When an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide, a primary amine with one carbon atom less than the original amide is produced. This degradation reaction is known as Hoffmann bromamide reaction. This reaction involves the migration of an alkyl or aryl group from the carbonyl carbon atom of the amide to the nitrogen atom. For example,
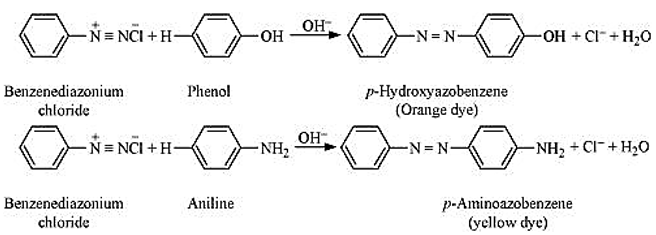
- Coupling reaction: The reaction of joining two aromatic rings through the - N = N - bond is known as coupling reaction. Benzene diazonium salt reacts with phenol or aromatic amines to form coloured azo compounds.
- Ammonolysis: When an alkyl or benzyl halide is allowed to react with an ethanolic solution of ammonia, it undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino (-NH2) group. This process of cleavage of the carbon-halogen bond is known as ammonolysis.
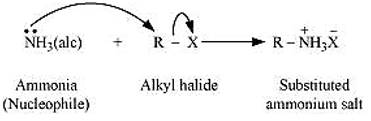
When this substituted ammonium salt is treated with a strong base such as sodium hydroxide, amine is obtained.
Though primary amine is produced as the major product, this process produces a mixture of primary, secondary and tertiary amines, and also a quaternary ammonium salt as shown.
- Carbylamine reaction: Carbylamine reaction is used as a test for the identification of primary amines. When aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide, carbylamines (or isocyanides) are formed. These carbylamines have very unpleasant odours. Secondary and tertiary amines do not respond to this test. For example,