Chemistry in Everyday Life (Not for Exams)-Test Papers
CBSE Test Paper-01
Class - 12 Chemistry (Chemistry in Everyday Life)
- The narrow spectrum antibiotic is
- Chloramphenicol
- Penicillin G
- Vancomycin
- Ofloxacin
- Use of aspartame is limited because
- it has a bad odour
- it is a high potency sweetener
- it adds calories to food
- it is unstable at cooking temperature
- Which of the following is not an antibiotic?
- Chloramphenicol
- Bithional
- Penicillin
- Sulphadiazine
- Tranquilizers act as antidepressant by
- Decreasing the degradation of noradrenaline
- Binding itself to noradrenaline
- Performing the function of neurotransmitters
- Increasing the degradation of noradrenaline
- Lather formed in shaving soaps is due to
- Sodium silicate
- Glycerol
- Borax
- Sodium rosinate
Which type of drugs belong to antimicrobial drugs?
What are tranquilizers?
What is the use of plant Rauwolfia serpentina in Ayurveda?
Describe Antiseptics giving suitable examples.
List two major classes of antibiotics and give one example of each class.
Why is bithional added to the toilet soap?
Low level of noradrenaline is the cause of depression. What types of drugs are needed to cure this problem? Name two drugs.
How are detergents classified?
- Give one example of each:
- Antacid
- Disinfectant
- Antiseptic
- Explain the following terms with suitable examples
- Cationic detergents
- Anionic detergents and
- Non-ionic detergents
CBSE Test Paper-01
Class - 12 Chemistry (Chemistry in Everyday Life)
Solutions
- (b) Penicillin G
Explanation: Those antibiotics which are effective mainly against Gram-positive or Gram-negative bacteria are narrow spectrum antibiotics. Penicillin G is narrow spectrum antibiotic as it works against only gram positive bacteria. - (d) it is unstable at cooking temperature
Explanation: Use of aspartame is limited to cold foods and soft drinks because it is unstable at cooking temperature. - (b) Bithional
Explanation: Antiseptics are the chemicals which either kill or prevent the growth of microorganisms. Bithionol (the compound is also called bithional) is added to soaps to impart antiseptic properties - (a) Decreasing the degradation of noradrenaline
Explanation: Transquilizers function by different mechanisms. For example, the antidepressant drugs inhibit the enzymes which catalyse the degradation of the neurotransmittor noradrenaline. If the enzyme is inhibited, this important neurotransmitter is slowly metabolised and can activate its receptor for longer periods of time, thus counteracting the effect of depression. - (d) Sodium rosinate
Explanation: Shaving soaps contain glycerol to prevent rapid drying. A gum called, rosin is added while making them. It forms sodium rosinate which lathers well. So lather forms in shaving soaps is due to Sodium rosinate. Antiseptic, antibiotics and disinfectants.
Tranquilizers are neurologically active drugs which are used for the treatment of stress and mild or even severe mental diseases.
It is used for reducing blood pressure.
Antiseptics: Those chemicals which kill or prevent the growth of micro-organism. Antiseptics could be applied to the living tissues such as wounds, cuts etc. Example - Dettol, Soframicine.
- Antibiotics may be defined as the sub-group of anti-infectives that are derived from bacterial sources and are used to treat bacterial infections.
An antibiotic may be classified basically as "narrow-spectrum" or "broad-spectrum" depending on the range of bacterial types that it affects.- Narrow-spectrum antibiotics are active against a selected group of bacterial types.e. g. Pencillin.
- Broad-spectrum antibiotics are active against a wider number of bacterial types and, thus, may be used to treat a variety of infectious diseases. e.g. chloramphenicol.
Bithional is added to the toilet soap to remove the bad odour produced by bacterial decomposition of organic matter on the skin. It acts as an antiseptic agent.
- Anti-depressant drugs are needed to counteract the effect of depression. These drugs inhibit enzymes catalysing the degradation of the neurotransmitter, noradrenaline. As a result, the important neurotransmitter is slowly metabolised and then it can activate its receptor for longer periods of time. Two anti-depressant drugs are:
- Iproniazid
- Phenelzine
- Synthetic detergents are classified as:
- Anionic detergents - these are sodium salts of sulphonated long chain alcohols or hydrocarbon. Here, the anionic part of the molecule is involved in the cleansing action. e.g. sodium lauryl sulphate.
- Cationic detergents – these are quaternary ammonium salts of amines with acetates, chlorides or bromides anions. e.g. cetyl trimethyl - ammonium bromide.
- Non - ionic detergents – these detergents do not contain any ion in their constitution. e.g. CH3(CH2)16COO(CH2CH2O)nCH2CH2OH.
- Ranitidine (Zantac)
- 1% Phenol
- Furacine, soframicine
- Cationic detergent: Cationic detergents are quaternary ammonium salts of acetates, chlorides, or bromides. These are called cationic detergents because the cationic part of these detergents contains a long hydrocarbon chain and a positive charge on the N atom.
For example: cetyltrimethylammonium bromide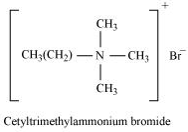
- Anionic detergents: Anionic detergents are of two types:
- Sodium alkyl sulphates: These detergents are sodium salts of long chain alcohols. They are prepared by first treating these alcohols with concentrated sulphuric acid and then with sodium hydroxide. Examples of these detergents include sodium lauryl sulphate (C11H23CH2OSO3-Na+) and sodium stearyl sulphate (C17H35CH2OSO3-Na+).
- Sodium alkylbenzenesulphonates: These detergents are sodium salts of long chain alkylbenzenesulphonic acids. They are prepared by Friedel-Crafts alkylation of benzene with long chain alkyl halides or alkenes. The obtained product is first treated with concentrated sulphuric acid and then with sodium hydroxide. Sodium 4-(1-dodecy) benzenesulphonate (SDS) is an example of anionic detergents.
- Non-ionic detergents: Molecules of these detergents do not contain any ions. These detergents are esters of alcohols having high molecular mass. They are obtained by reacting polyethylene glycol and stearic acid.
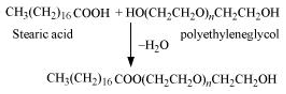
- Cationic detergent: Cationic detergents are quaternary ammonium salts of acetates, chlorides, or bromides. These are called cationic detergents because the cationic part of these detergents contains a long hydrocarbon chain and a positive charge on the N atom.