Haloalkanes and Haloarenes-Test Papers
CBSE Test Paper-01
Class - 12 Chemistry (Haloalkanes and Haloarenes)
- What is inversion of configuration?
- secondary butyl chloride
- w-butyI bromide
- tert-butyl chloride
- iso-butyl iodide
- Bromomethane, Chloromethane, Dibromomethane. 1 – Chloropropane, Isopropyl chloride, 1 – Chlorobutaneare all
- Completely soluble in organic solvents
- Slightly soluble in organic solvents
- Insoluble in organic solvents
- Completely soluble in water
- Triiodomethane (Iodoform) is
- Pesticide
- Refrigerant
- antiseptic drug
- degreasing agent
- Reactions with iodine in preparation of aryl iodide from arenes require the presence of
- diazonium salt
- an oxidizing agent
- a reducing agent
- ZnCl2 catalyst
- Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and paranitroanisole
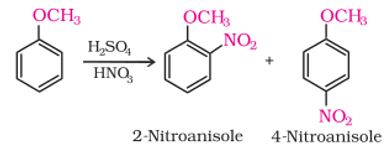
- None of these
- minor product is orthonitroanisole
- major product is paranitroanisole
- ortho and para in equal amounts.
- What is meant by axis of symmetry?
- Give IUPAC names of:
- Give IUPAC name of:
- Give the structure of 1,3-dichloro -2-(bromomethyl) propane
- Complete the following reaction equation:
- C6H5N2+Cl+ + KI ..........
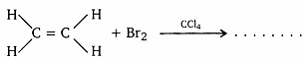
- Write the structure of the major organic product in the following reaction:
CH3CH2CH2OH + SOCl2 - A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
- Write the structural formula of the organic compounds A and B in the following sequence of reaction.
- Point out the difference between:
- Chirality and chiral centre.
- Diastereoisomers and Enantiomers.
- Explain why
- the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
- alkyl halides, though polar, are immiscible with water?
- Grignard reagents should be prepared under anhydrous conditions?
CBSE Test Paper-01
Class - 12 Chemistry (Haloalkanes and Haloarenes)
Solutions
- (a) secondary butyl chloride
Explanation: Secondary butyl chloride is optically active because it has chiral carbon atom marked* - (a) Completely soluble in organic solvents
Explanation: These all are covalent compounds hence are soluble in organic solvents. - (c) antiseptic drug
Explanation: The compound finds small scale use as a disinfectant.Around the beginning of the 20th century it was used in medicine as a healing and antiseptic dressing for wounds and sores, although this use is now superseded by superior anticeptics. - (b) an oxidizing agent
Explanation: Reactions with iodine are reversible in nature and require the presence of an oxidising agent (HNO3, HIO4) to oxidise the HI formed during iodination. - (c) major product is paranitroanisole
Explanation: OCH3 is activator and o/p director out of which para is major product. - It is an imaginary axis around which if the compound is rotated by a minimum angle of rotation, it gives back the original molecule with same configuration.
- 1-Chloropropan-2-ol
- In writing the IUPAC name, we first count the number of C atoms in the longest C chain (parent chain) and assign the locants according to the functional groups attached. Here as we can see the longest chain contains 4 C and it is an alkane, so we name it butane. The -Br (bromo) group is attached at position 1 and 3. So the name of the compound is 1,3-dibromobutane.
- We can understand from the name that the longest C chain contains 3 C (as it is propane). Also at positions 1 and 3, -Cl is attached and at position 2, bromomethyl group i.e. -CH2Br is attached. So the structure of the given compound must be:
- C6H5N2+Cl- + KI C6H5I + KCI + N2
- A hydrocarbon with the molecular formula, C5H10 belongs to the group with a general molecular formula CnH2n.Therefore, it may either be an alkene or a cycloalkane. Since hydrocarbon does not react with chlorine in the dark, it cannot be an alkene. Thus, it should be a cycloalkane. Further, the hydrocarbon gives a single monochloro compound, C5H9Cl by reacting with chlorine in bright sunlight. Since a single monochloro compound is formed, the hydrocarbon must contain H-atoms that are all equivalent. Also, as all H-atoms of a cycloalkane are equivalent, the hydrocarbon must be a cycloalkane. Hence, the said compound is cyclopentane.
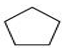
Cyclopentane (C5H10) The reactions involved in the question are: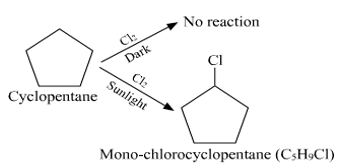
- CH3-CH=CH-CH3
Thus A is but-2-ene and B is 2,3-dibromobutane - Chirality: Chirality is the property of a molecule, containing a carbon attached to four different groups, having a non-superimposable mirror image.
Chiral centre: The carbon which is attached to four different groups is called chiral centre. - Diastereoisomers: Those pairs of stereoisomers which are not mirror images of each other. They differ in optical rotation.
Enantiomers: They are non-superimposable mirror images of each other. They have optical rotation equal in magnitude but opposite in sign.
- Chirality: Chirality is the property of a molecule, containing a carbon attached to four different groups, having a non-superimposable mirror image.
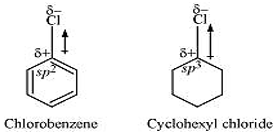
In chlorobenzene, the Cl-atom is linked to a sp2 hybridized carbon atom. In cyclohexyl chloride, the Cl-atom is linked to a sp3 hybridized carbon atom. Now, sp2 hybridized carbon has more s-character than sp3 hybridized carbon atom. Therefore, the former is more electronegative than the latter. Therefore, the density of electrons of C - Cl bond near the Cl-atom is less in chlorobenzene than in cyclohexyl chloride. Moreover, the - R effect of the benzene ring of chlorobenzene decreases the electron density of the C - Cl bond near the Cl-atom. As a result, the polarity of the C - Cl bond in chlorobenzene decreases. Hence, the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.- To be miscible with water, the solute-water force of attraction must be stronger than the solute-solute and water-water forces of attraction. Alkyl halides are polar molecules and so held together by dipole-dipole interactions. Similarly, strong H-bonds exist between the water molecules. The new force of attraction between the alkyl halides and water molecules is weaker than the alkyl halide-alkyl halide and water-water forces of attraction. Hence, alkyl halides (though polar) are immiscible with water.
- Grignard reagents are very reactive. In the presence of moisture, they react to give alkanes.
Therefore, Grignard reagents should be prepared under anhydrous conditions.