Solid State-Solutions
CBSE Class 12 Chemistry
NCERT Solutions
Chapter - 01
The Solid State
1. Define the term 'amorphous'. Give a few examples of amorphous solids.
Ans. Amorphous solids are the solids whose constituent particles are irregularly arranged and have short range order i.e. a regular and periodically repeating pattern is observed over short distances only. Such portions are scattered and in between the arrangement is disordered. . These solids are isotropic in nature and melt over a range of temperature. Therefore, amorphous solids are sometimes called pseudo solids or super cooled liquids. They do not have definite heat of fusion. When cut with a sharp-edged tool, they cut into two pieces with irregular surfaces. Examples of amorphous solids include glass, rubber, and plastic.
2. What makes a glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
Ans. The arrangement of the constituent particles makes glass different from quartz. In glass, the constituent particles have short-range order i.e. a regular and periodically repeating pattern is observed over short distances only, but in quartz, the constituent particles have long range order i.e. there is a regular pattern of arrangement of particles which repeats itself periodically over the entire crystal. So quartz is crystalline whereas glass is an amorphous solid.
Quartz can be converted into glass by heating and then cooling it rapidly.
3. Classify the following as amorphous or crystalline solids:
Polyurethane, naphthalene, benzoic acid, teflon, potassium nitrate, cellophane, polyvinyl chloride, fibre glass, copper.
Ans. Amorphous solids: An amorphous solid consists of particles of irregular shape. Polyurethane, teflon, cellophane, polyvinyl chloride and fibre glass fall in this category.
Crystalline solids: A crystalline solid usually consists of a large number of small crystals, each of them having a definite characteristic geometrical shape. Naphthalene, benzoic acid, potassium nitrate and copper fall in this category.
4. (i) What is meant by the term 'coordination number'?
(ii) What is the coordination number of atoms:
(a) in a cubic close-packed structure?
(b) in a body-centred cubic structure?
Ans. (i) The number of nearest neighbours of any constituent particle present in the crystal lattice is called its coordination number.
(ii) The coordination number of atoms
(a) in a cubic close-packed structure is 12, and
(b) in a body-centred cubic structure is 8
5. How can you determine the atomic mass of an unknown metal if you know its density and the dimension of its unit cell? Explain.
Ans. By knowing the density of an unknown metal and the dimension of its unit cell, the atomic mass of the metal can be determined.
Let ‘a’ be the edge length of a unit cell of a crystal, ‘d’ be the density of the metal, ‘m’ be the atomic mass of one atom of the metal and ‘z’ be the number of atoms in the unit cell.
Now, density of the unit cell =Mass of the unit cell/ Volume of the unit cell
d= ……………………(i)
[Since mass of the unit cell = Number of atoms in the unit cell x mass of one atom and mass of one atom = atomic mass / NA]
[Volume of the unit cell = (Edge length of the cubic unit cell)3]
From equation (i), we have :
m = ……………………..(ii)
if it is not a cubic unit cell then dimensions of unit cell will be a, b, c and volume of unit cell = a b c
So, m=
6. 'Stability of a crystal is reflected in the magnitude of its melting point'. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Ans. Higher the melting point, greater is the intermolecular force of attraction and greater is the stability. A substance with higher melting point is more stable than a substance with lower melting point.
The melting points of the given substances are:
Solid water = 273 K
Ethyl alcohol = 158.8 K
Diethyl ether = 156.85 K
Methane = 89.34 K
Now, on observing the values of the melting points, it can be said that among the given substances, the intermolecular force in solid water is the strongest and that in methane is the weakest. This is because of existence of hydrogen bonding (strong interaction) in solid water (ice) because of the polarity of O-H bond due to difference in electronegativity of O and H. In methane, only weak vander waal forces exist between molecules because methane is a non-polar molecule due to very less difference in electronegativity of C and H.
7. How will you distinguish between the following pairs of terms:
(i) Hexagonal close-packing and cubic close-packing?
(ii) Crystal lattice and unit cell?
(iii) Tetrahedral void and octahedral void?
Ans. A 2-D hexagonal close-packing contains two types of triangular voids (shaded a and b) as shown in figure 1. Let us call this 2-D structure as layer A. Now, particles are kept in the voids present in layer A (it can be easily observed from figures 2 and 3 that only one of the voids will be occupied in the process, i.e., either a or b). Let us call the particles or spheres present in the voids of layer A as layer B. Now, two types of voids are present in layer B (c and d). Unlike the voids present in layer A, the two types of voids present in layer B are not similar. Void c is surrounded by 4 spheres and is called the tetrahedral void. Void d is surrounded by 6 spheres and is called the octahedral void.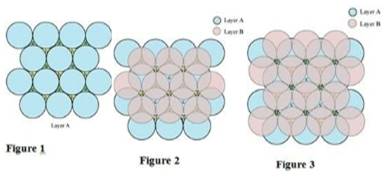
Now, the next layer can be placed over layer B in 2 ways.
Case 1: When the third layer (layer C) is placed over the second one (layer B) in such a manner that the spheres of layer C occupy the tetrahedral voids c.
In this case, we get hexagonal close-packing. This is shown in figure 4.1 and 4.2. In figure 4.1, layer B is present over the voids a and layer C is present over the voids c. In figure 4.2, layer B is present over the voids b and layer C is present over the voids c. It can be observed from the figure that in this arrangement, the spheres present in layer C are present directly above the spheres of layer A. Hence, we can say that the layers in hexagonal close-packing are arranged in an ABAB…..pattern.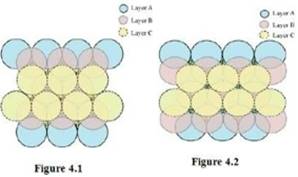
Case 2: When the third layer (layer C) is placed over layer B in such a manner that the spheres of layer C occupy the octahedral voids d.
In this case, we get cubic close-packing. In figure 5.1, layer B is present over the voids a and layer C is present over the voids d. In figure 5.2, layer B is present over the voids b and layer C is present over the voids d. It can be observed from the figure that the arrangement of particles in layer C is completely different from that in layers A or B. When the fourth layer is kept over the third layer, the arrangement of particles in this layer is similar to that in layer A. Hence, we can say that the layers in cubic close-packing are arranged in an ABCABC…..pattern.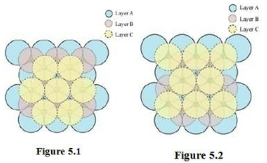
8. How many lattice points are there in one unit cell of each of the following lattice?
(i) Face-centred cubic
(ii) Face-centred tetragonal
(iii) Body-centred
Ans. (i) There are 14 (8 from the corners + 6 from the faces) lattice points in face-centred cubic lattice.
(ii) There are 14 (8 from the corners + 6 from the faces) lattice points in face-centred tetragonal lattice.
(iii) There are 9 (1 from the centre + 8 from the corners) lattice points in body-centred cubic lattice.
9. Explain
(i) The basis of similarities and differences between metallic and ionic crystals.
(ii) Ionic solids are hard and brittle.
Ans. (i) The basis of similarities between metallic and ionic crystals is that both these crystal types are held together by the electrostatic force of attraction. In metallic crystals, the electrostatic force of attraction acts between the positive metal ions (Kernels) and the electrons. In ionic crystals, it acts between the oppositely-charged ions (cation and anion). Hence, both have high melting points.
The basis of differences between metallic and ionic crystals is that in metallic crystals, the electrons are free to move and are evenly spread out throughout the crystal. Each metal atom contributes one or more electrons towards this sea of mobile electrons. These free and mobile electrons are responsible for high electrical and thermal conductivity of metals. However, in ionic crystals, the ions are not free to move. As a result, they cannot conduct electricity in solid state i.e. they are electrical insulators. However, in molten state or in aqueous solution, they do conduct electricity because the ions become free to move in that case.
(ii) The constituent particles of ionic crystals are cations and anions. These ions are held together in three-dimensional arrangements by the coulombic (electrostatic) force of attraction. Since the electrostatic force of attraction is very strong, the charged ions are held in fixed positions in the lattice structure. This is the reason why ionic crystals are hard and brittle.
10. Calculate the efficiency of packing in case of a metal crystal for
(i) simple cubic
(ii) body-centred cubic
(iii) face-centred cubic (with the assumptions that atoms are touching each other).
Ans. (i) Simple cubic:
In a simple cubic lattice, the particles are located only at the corners of the cube and touch each other along the edge.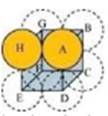
Let the edge length of the cube be ‘a’ and the radius of each particle be r.
So, we can write: a = 2r
Now, volume of the cubic unit cell = a3
= (2r)3 = 8r3
We know that the number of particles per unit cell is 1.
Therefore, volume occupied by atoms in unit cell =
Hence, packing efficiency = (volume occupied by particles in unit cell / volume of cubic unit cell) x 100
=
and a= 2r
=52.4%
(ii) Body-centred cubic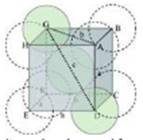
It can be observed from the above figure that the atom at the centre is in contact with the other two atoms diagonally arranged.
From ΔFED, we have:
b2=a2+a2
⇒b2=2a2
⇒b=1.414a
Again, from AFD, we have:
c2=a2+b2
⇒c2=a2+2a2
⇒c2=3a2 (Since b2=2a2)
c =1.73 a
Let the radius of the atom be r.
Length of the body diagonal, c= 4r = 1.73
a = 4r
Volume of the cube, a3 =
A body-centred cubic lattice contains 2 atoms.
So, volume of the occupied by atoms in cubic lattice =
Therefore, Packing efficiency = Volume occupied by two spheres in the unit cell / Total volume of the unit cell 100
= [using a3= ]
Therefore, Packing efficiency = 68 %
(iii) Face-centred cubic
Let the edge length of the unit cell be ‘a’ and the length of the face diagonal AC be b.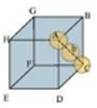
From ΔABC, we have:
AC2 = BC2 + AB2
b2 = a2+ a2
b = 1.414 a
Let r be the radius of the atom.
Now, from the figure, it can be observed that:
b=4r
Now, volume of the cube = a3
We know that the number of atom per unit cell is 4.
So, volume occupied by atoms of unit cell=
Packing Efficiency =
Packing efficiency =
and 1.414a = 4r
= 74%
11. Silver crystallizes in fcc lattice. If edge length of the cell is 4.07 10-8 cm and density is 10.5 g cm-3, calculate the atomic mass of silver.
Ans. It is given that the edge length, a= 4.07 7 10-8 cm
Density, d = 10.5 g cm-3
As the lattice is fcc type, the number of atoms per unit cell, z= 4
We also know that, NA=6.023 1023
Using the relation:
d=
m =
= 107.13 g mol-1
12. A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body-centre. What is the formula of the compound? What are the coordination numbers of P and Q?
Ans. It is given that the atoms of Q are present at the corners of the cube.
Therefore, number of atoms of Q in one unit cell = 8 1/8 = 1
It is also given that the atoms of P are present at the body-centre.
Therefore, number of atoms of P in one unit cell = 1
This means that the ratio of the number of P atoms to the number of Q atoms, P:Q = 1:1
Hence, the formula of the compound is PQ.
The coordination number of both P and Q is 8.
13. Niobium crystallises in body–centred cubic structure. If density is 8.55 g cm-3, calculate atomic radius of niobium using its atomic mass 93 u.
Ans. It is given that the density of niobium, d= 8.55 g cm-3
Atomic mass, M = 93 g mol-1
As the lattice is bcc type, the number of atoms per unit cell, z= 2
We also know that, NA=6.023 1023
Applying the relation:
d=
a3 =
= 3.612 10-23 cm3
So, a = 3.306 10-8 cm
For body-centred cubic unit cell:
4r =1.73a
r =
= 1.432 10-8 cm
= 14.32 10-9 cm
14. If the radius of the octachedral void is r and radius of the atoms in close packing is R, derive relation between r and R.
Ans.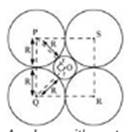
A sphere with centre O, is fitted into the octahedral void as shown in the above figure. It can be observed from the figure that POQ is right-angled
Now, applying Pythagoras theorem, we can write:
PQ2 = PO2 + OQ2
(2R)2 = (R + r)2 + (R + r)2
(2R)2 = 2(R + r)2
2R2 = (R + r)2
r = 0.414 R
15. Copper crystallises into a fcc lattice with edge length 3.61 10-8 cm. Show that the calculated density is in agreement with its measured value of 8.92 g cm-3.
Ans. Edge length, a = 3.61 10-8 cm
As the lattice is fcc type, the number of atoms per unit cell, z= 4
Atomic mass, M = 63.5 g mol-1
We also know that, NA = 6.023 1023
Applying the relation:
d=
=
=8.96 g cm-3
The measured value of density is given as 8.92 g cm-3. Hence, the calculated density 8.97 g cm-3 is in agreement with its measured value.
16. Analysis shows that nickel oxide has the formula Ni0.98O1.00. What fractions of nickel exist as Ni2+ and Ni3+ ions?
Ans. The formula of nickel oxide is Ni0.98O1.00.
Therefore, the ratio of the number of Ni atoms to the number of O atoms, Ni: O = 0.98 : 1.00 = 98 : 100
Now, total charge on 100 O2- ions = 100(-2)= -200
Let the number of Ni2+ions be x.
So, the number of Ni3+ions is 98 –x.
Now, total charge on Ni2+ions = x(+2)
= +2x
And, total charge on Ni3+ ions = (98 –x)(+3)
= 294 –3x
Since, the compound is neutral, we can write:
2x+ (294 –3x) + (– 200) = 0
⇒ –x + 94 = 0
⇒ x = 94
Therefore, number of Ni2+ ions = 94
And, number of Ni3+ ions = 98 – 94 = 4
Hence, fraction of nickel that exists as Ni2+= 94/98 = 0.959
And, fraction of nickel that exists as Ni3+ = 4/98 = 0.041
Alternatively, fraction of nickel that exists as Ni3+ = 1 – 0.959 = 0.041
17. What is a semiconductor? Describe the two main types of semiconductors and contrast their conduction mechanism.
Ans. Semiconductors are substances having conductance in the intermediate range of 10–6 to 104 ohm–1m–1. In semiconductors, the gap between the valence band and conduction band is small and therefore, some electrons may jump to conduction band and show some conductivity. The conductivity of these intrinsic semiconductors is too low to be of practical use. Their conductivity is increased by adding an appropriate amount of suitable impurity. This process is called doping. Doping can be done with an impurity which is electron rich or electron deficient as compared to the intrinsic semiconductor silicon or germanium. Such impurities introduce electronic defects in them.
The two main types of semiconductors are:
(i) n-type semiconductor: The semiconductor whose increased conductivity is a result of negatively-charged electrons is called an n-type semiconductor. When the crystal of a group 14 element such as Si or Ge is doped with a group 15 element such as P or As, an n-type semiconductor is generated.
Si and Ge have four valence electrons each. In their crystals, each atom forms four covalent bonds. On the other hand, P and As contain five valence electrons each. When Si or Ge is doped with P or As, the latter occupies some of the lattice sites in the crystal. Four out of five electrons are used in the formation of four covalent bonds with four neighbouring Si or Ge atoms. The remaining fifth electron becomes delocalised and increases the conductivity of the doped Si or Ge.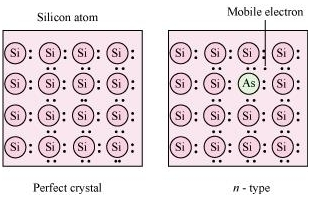
(ii) p-type semiconductor: The semiconductor whose increased in conductivity is a result of electron hole is called a p-type semiconductor. When a crystal of group 14 elements such as Si or Ge is doped with a group 13 element such as B, Al, or Ga (which contains only three valence electrons), a p-type of semiconductor is generated.
When a crystal of Si is doped with B, the three electrons of B are used in the formation of three covalent bonds and an electron hole is created. An electron from the neighboring atom can come and fill this electron hole, but in doing so, it would leave an electron hole at its original position. The process appears as if the electron hole has moved in the direction opposite to that of the electron that filled it. Therefore, when an electric field is applied, electrons will move toward the positively-charged plate through electron holes. However, it will appear as if the electron holes are positively-charged and are moving toward the negatively- charged plate.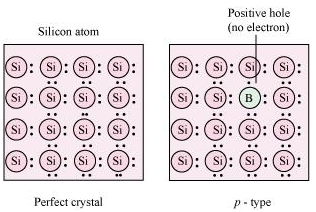
18. Non-stoichiometric cuprous oxide, Cu2O can be prepared in laboratory. In this oxide, copper to oxygen ratio is slightly less than 2:1. Can you account for the fact that this substance is a p-type semiconductor?
Ans. In the cuprous oxide (Cu2O) prepared in the laboratory, copper to oxygen ratio is slightly less than 2:1. This means that the number of Cu+ ions is slightly less than twice the number of O2− ions. This is because some Cu+ ions have been replaced by Cu2+ ions. Every Cu2+ ion replaces two Cu+ ions, thereby creating holes. As a result, the substance conducts electricity with the help of these positive holes. Hence, the substance is a p-type semiconductor.
19. Ferric oxide crystallises in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Ans. Let the number of oxide O2- ions be x.
So, number of octahedral voids = x
It is given that two out of every three octahedral holes are occupied by ferric ions.
So, number of ferric (Fe3+)ions= 2/3x
Therefore, ratio of the number of Fe3+ions to the number of O2− ions, Fe3+: O2−
=2 / 3x : x = 2/3 : 1 = 2 : 3
Hence, the formula of the ferric oxide is Fe2O3.
20. Classify each of the following as being either a p-type or an n-type semiconductor:
(i) Ge doped with In
(ii) B doped with Si.
Ans. (i) When Ge (a group 14 element) having four valence electrons is doped with In (a group 13 element) which contains only three valence electrons. The place where the fourth valence electron is missing is called an electron hole or electron vacancy. An electron from a neighbouring atom can come and fill the electron hole, but in doing so it would leave an electron hole at its original position. The semiconductor thus generated will be a p-type semiconductor.
(ii) B (a group 13 element) having three valence electrons is doped with Si (a group 14 element) which has four valence electrons. Three out of four electrons are used in the formation of three covalent bonds with the three neighbouring Boron atoms. The fourth electron is extra and becomes delocalised. These delocalised electrons increase the conductivity of doped Boron. Thus, a electron will be created and the semiconductor generated will be a n-type semiconductor.
21. Gold (atomic radius = 0.144 nm) crystallises in a face-centred unit cell. What is the length of a side of the cell?
Ans. For a face-centred unit cell 4r=1.414a
It is given that the atomic radius, r= 0.144 nm
So, 4(0.144)/1.414 = a
= 0.407 nm
Hence, length of a side of the cell = 0.407 nm
22. In terms of band theory, what is the difference
(i) Between a conductor and an insulator
(ii) Between a conductor and a semiconductor
Ans. (i) The valence band of a conductor is partially-filled or it overlaps with a higher energy, unoccupied conduction band.
On the other hand, in the case of an insulator, the valence band is fully- filled and there is a large gap between the valence band and the conduction band.
(ii) In the case of a conductor, the valence band is partially-filled or it overlaps with a higher energy, unoccupied conduction band. So, the electrons can flow easily under an applied electric field.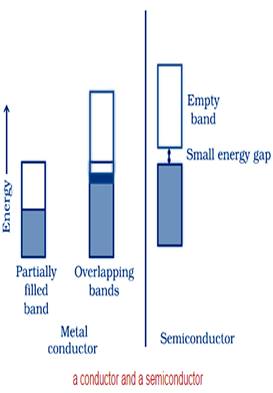
On the other hand, the valence band of a semiconductor is filled and there is a small gap between the valence band and the next higher conduction band. Therefore, some electrons can jump from the valence band to the conduction band and conduct electricity.
23. Explain the following terms with suitable examples:
(i) Schottky defect
(ii) Frenkel defect
(iii) Interstitials and
(iv) F-centres
Ans. (i) Schottky defect: Schottky defect is basically a vacancy defect shown by ionic solids. In this defect, an equal number of cations and anions are missing to maintain electrical neutrality. It decreases the density of a substance. Significant number of Schottky defects is present in ionic solids. For example, in NaCl, there are approximately 106 Schottky pairs per cm3 at room temperature. Ionic substances containing similar-sized cations and anions show this type of defect. For example: NaCl, KCl, CsCl, AgBr, etc.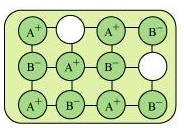
(ii) Frenkel defect: Ionic solids containing large differences in the sizes of ions show this type of defect. When the smaller ion (usually cation) is dislocated from its normal site to an interstitial site, Frenkel defect is created. It creates a vacancy defect as well as an interstitial defect. Frenkel defect is also known as dislocation defect. Ionic solids such as AgCl, AgBr, AgI, and ZnS show this type of defect.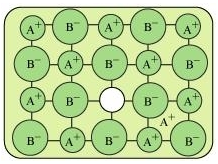
(iii) Interstitials: Interstitial defect is shown by non-ionic solids. This type of defect is created when some constituent particles (atoms or molecules) occupy an interstitial site of the crystal. The density of a substance increases because of this defect.
(iv) F-centres: When the anionic sites of a crystal are occupied by unpaired electrons, the ionic sites are called F-centres. These unpaired electrons impart colour to the crystals. For example, when crystals of NaCl are heated in an atmosphere of sodium vapour, the sodium atoms are deposited on the surface of the crystal. The Cl ions diffuse from the crystal to its surface and combine with Na atoms, forming NaCl. During this process, the Na atoms on the surface of the crystal lose electrons. These released electrons diffuse into the crystal and occupy the vacant anionic sites, creating F-centres.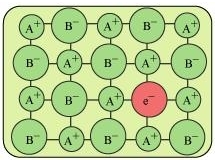
24. Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 125 pm.
(i) What is the length of the side of the unit cell?
(ii) How many unit cells are there in 1.00 cm3 of aluminium?
Ans. (i) For cubic close-packed structure: 4r=1.414a
so, a= 4(125pm)/1.414
= 353.55 pm
= 354 pm (approximately)
(ii) Volume of one unit cell = (354 pm)3
= 4.4107pm3
=4.4107 10-30 cm3
= 4.4 10-23 cm3
so in 4.4 10-23 cm3 we have 1 unit cell
therefore in 1cm3 we have = 1/(4.4 10 -23 ) unit cell
= 2.271022
25. If NaCl is doped with 10-3mol% of SrCl2, what is the concentration of cation vacancies?
Ans. It is given that NaCl is doped with 10-3% of SrCl2.
This means that 100 mol of NaCl is doped with 10−3mol of SrCl2.
Therefore, 1mol of NaCl is doped with = 10-3/100 mole of SrCl2
= 10-5 mol of SrCl2
Cation vacancies produced by one Sr2+ ion = 1
Therefore, Concentration of the cation vacancies
Produced by 10-5mol of Sr2+ ions =
Hence, the concentration of cation vacancies created by SrCl2 is
26. Explain the following with suitable examples:
(i) Ferromagnetism
(ii) Paramagnetism
(iii) Ferrimagnetism
(iv) Antiferromagnetism
(v) 12-16 and 13-15 group compounds.
Ans. (i) Ferromagnetism: The substances that are strongly attracted by a magnetic field are called ferromagnetic substances. Besides strong attractions, ferromagnetic substances can be permanently magnetised. Some examples of ferromagnetic substances are iron, cobalt, nickel, gadolinium, and CrO2.
In solid state, the metal ions of ferromagnetic substances are grouped together into small regions called domains and each domain acts as a tiny magnet. In an un-magnetised piece of a ferromagnetic substance, the domains are randomly-oriented and so, their magnetic moments get cancelled. However, when the substance is placed in a magnetic field, all the domains get oriented in the direction of the magnetic field. As a result, a strong magnetic effect is produced. This ordering of domains persists even after the removal of the magnetic field. Thus, the ferromagnetic substance becomes a permanent magnet.
Schematic alignment of magnetic moments in ferromagnetic substances
(ii) Paramagnetism: The substances that are attracted by a magnetic field are called paramagnetic substances.
Paramagnetic substances get magnetised in a magnetic field in the same direction, but lose magnetism when the magnetic field is removed. To undergo paramagnetism, a substance must have one or more unpaired electrons. This is because the unpaired electrons are attracted by a magnetic field, thereby causing paramagnetism.
(iii) Ferrimagnetism: The substances in which the magnetic moments of the domains are aligned in parallel and anti-parallel directions, in unequal numbers, are said to have ferrimagnetism. Examples include Fe3O4(magnetite), ferrites such as MgFe2O4 and ZnFe2O4.
Ferrimagnetic substances are weakly attracted by a magnetic field as compared to ferromagnetic substances. On heating, these substances become paramagnetic.
Schematic alignment of magnetic moments in ferrimagnetic substances
(iv) Antiferromagnetism: Antiferromagnetic substances have domain structures similar to ferromagnetic substances, but are oppositely-oriented. The oppositely-oriented domains cancel out each other's magnetic moments.
Schematic alignment of magnetic moments in antiferromagnetic substances
(v) 12-16 and 13-15 group compounds: The 12-16 group compounds are prepared by combining group 12 and group 16 elements and the 13-15 group compounds are prepared by combining group 13 and group 15 elements. These compounds are prepared to stimulate average valence of four as in Ge or Si. Indium (III) antimonide (IrSb), aluminium phosphide (AlP), and gallium arsenide (GaAs) are typical compounds of groups 13-15. GaAs semiconductors have a very fast response time and have revolutionised the designing of semiconductor devices. Examples of group 12-16 compounds include zinc sulphide (ZnS), cadmium sulphide (CdS), cadmium selenide (CdSe), and mercury (II) telluride (HgTe). The bonds in these compounds are not perfectly covalent. The ionic character of the bonds depends on the electronegativities of the two elements.