Surface Chemistry-Test Papers
CBSE Test Paper-01
Class - 12 Chemistry (Surface Chemistry)
- The size of the particle is enough to scatter light and exhibits Tyndall effect
- 2000 - 4000 nm
- 200 - 2000 nm
- 1 - 100 nm
- 200 - 400 nm
- Which of the following statement is true about Langmuir’s adsorption?
- It occurs at low temperature
- It has a reversible temperature
- It is not specific in nature
- It forms a monolayer
- Micelles are:
- Ideal solution
- Associated colloids
- Adsorbed solution
- Emulsion cum gel
- Ammonia is adsorbed on activated charcoal to a larger extent as compare to CO because
- Ammonia reacts with oxygen
- Ammonia exist in liquid state
- CO reacts with oxygen
- Ammonia is more easily liquefiable
- Which of the following metal solution cannot be prepared by Bredig’s arc method?
- Platinum
- Copper
- Gold
- Potassium
- Why is the adsorption always exothermic?
- Compare the coagulating power of AlCl3 with that of NaCl. Given that their coagulation values are 0.093 and 52 respectively.
Give two examples of an associated colloids.
How is a colloidal solution purified by dialysis?
Name two industrial process in which heterogeneous catalysts are employed.
What do you understand by activation of adsorbent? How is it achieved?
What are the two classes of emulsions? Give one example of each class. State one activity to test the type of an emulsion.
- Write dispersion medium & dispersed phase of following colloid systems:
- An aerosol
- An emulsion
- A hydrosol
- How are colloids classified on the basis of:
- Physical states of components
- Nature of dispersion medium
- Interaction between dispersed phase and dispersion medium?
Give four examples of heterogeneous catalysis.
CBSE Test Paper-01
Class - 12 Chemistry (Surface Chemistry)
Solutions
- 1 - 100 nm
Explanation: Colloids have an intermediate size between suspension and true solutions. The particle size is enough to scatter light and exhibits Tyndall effect.
- 1 - 100 nm
- It forms a monolayer
Explanation: Langmuir isotherm assume monolayer formation only.
- It forms a monolayer
- Associated colloids
Explanation: Micelles are chemical structures formed with both hydrophilic (they’ll mix into water) and hydrophobic (they don’t mix into water). Also called as Associated colloids. In the general case, micelles are formed when there is an ideal temperature in the medium (called the Kraft temperature) and a certain concentration of electrolytes (called the CMC: Critical Micelle Concentration) in the medium.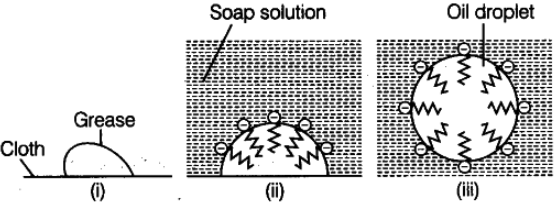
- Grease or oil on surface of cloth.
- Stearate ions arranged around the grease or oil droplet.
- Grease or oil droplet surrounded by stearate ions (ionic micelle formed).
- Associated colloids
- Ammonia is more easily liquefiable
Explanation: Ammonia is easily liquefiable because of H- bonding interactions.
- Ammonia is more easily liquefiable
- Potassium
Explanation: Potassium due to very high reactivity, cannot be used here.
- Potassium
- In adsorption process, there is always a decrease in residual forces of the surface i.e., there is decrease in surface energy which appears as heat. Hence, adsorption is exothermic in nature.
Thus, AlCl3 has 559 times greater coagulating power than NaCl.- Soaps and detergents are associated colloids.
- Dialysis is a process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane. A bag of suitable membrane containing the colloidal solution is suspended in a vessel through which fresh water is continuously flowing. The molecules and ions diffuse through membrane into outer water and pure colloidal solution is left behind.
- Contact process: 2SO2(g) + O2(g) 2SO3(g)
Ostwald process: 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g) - By activating an adsorbent, we tend to increase the adsorbing power (adsorbing sites) of an adsorbent. Some ways to activate an adsorbent are:
- By increasing the surface area of the adsorbent. This can be done by breaking it into smaller pieces or powdering it.
- Some specific treatments can also lead to the activation of the adsorbent. For example, wood charcoal is activated by heating it between 650 K and 1330 K in vacuum or air. It expels all the gases absorbed or adsorbed and thus, creates a space for adsorption of gases.
- The two types of emulsions are:
- Oil-in water type: In this type small droplets of an oil are dispersed in water. For example: milk, cod liver oil.
- Water in oil type: In this type which water droplets are dispersed in oil medium. For example, butter.
Colloid system Dispersion medium Dispersed phase Example (i) An aerosol Gas Solid or liquid Dust particles (ii) An emulsion Liquid Liquid Milk (iii)A hydrosol Water Solid Starch in water Colloids can be classified on various bases:
- On the basis of the physical state of the components (by components we mean the dispersed phase and dispersion medium). Depending on whether the components are solids, liquids, or gases, we can have eight types of colloids.
- On the basis of the dispersion medium, sols can be divided as:
Dispersion medium Name of sol Water Aquasol or hydrosol Alcohol Alcosol Benzene Benzosol Gases Aerosol - On the basis of the nature of the interaction between the dispersed phase and dispersion medium, the colloids can be classified as lyophilic (solvent attracting) and lyophobic (solvent repelling).
- Oxidation of sulphur dioxide to form sulphur trioxide. In this reaction, Pt acts as a catalyst.
- Formation of ammonia by the combination of dinitrogen and dihydrogen in the presence of finely divided iron catalyst.
This process is called the Haber's process. - Oswald's process: Oxidation of ammonia into nitric oxide in the presence of platinum gauze.
- Hydrogenation of vegetable oils in the presence of finely divided Ni catalyst.
- Oxidation of sulphur dioxide to form sulphur trioxide. In this reaction, Pt acts as a catalyst.