Biomolecules-Solutions
CBSE Class 12 Chemistry
NCERT Solutions
Chapter - 14
Biomolecules
NCERT INTEXT QUESTIONS.
14.1 Glucose or sucrose are soluble in water but cyclohexane and benzene (simple six membred ring compounds) are insoluble in water Explain.
Ans. The solubility of a solute in a given solvent follows the rule ‘ Like dissolves like’. Glucose contains five and sucrose contains eight -OH groups. These -OH groups form H-bonds with water. As a result of this extensive inter molecular H-bonding, glucose and sucrose are soluble in water. On the other hand, benzene and cyclohexane do not contain -OH bonds and hence do not form H-bonds with water. Moreover, they are non-polar molecules and hence do not dissolve in polar water molecules.
14.2 What are the expected products of hydrolysis of lactose?
Ans. Lactose being a disaccharide gives two molecules of monosaccharides Le. one molecule each of D-(+) – glucose and D-(+)-galactose.
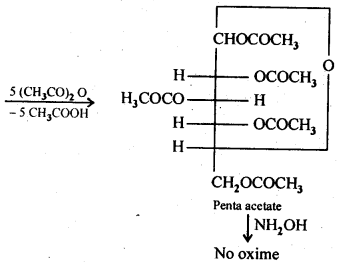
14.3 How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Ans. The cyclic hemiacetal form of glucose contains an -OH group at C-l which gets hydrolysed in aqueous solution to produce open chain aldehydic form which then reacts with NH2OH -to form corresponding oxide. Thus, glucose contains an aldehydic group. However, when glucose is reacted with acetic anhydride, the -OH group at C-l along with the other -OH groups at C-2, C-3, C-4 and C-6 form a pentaacetate.
Since the penta acetate of 1 glucose does not contain a free -OH group at C-l, it cannot get hydrolysed in aqueous solution to produce open chain aldehydic form and hence glucose pentaacetate does not react with NH2OH to form glucose oxime. The reactions are shown as: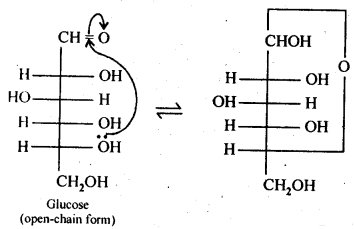
14.4 The melting points and solubility of amino acids in water are generally higher than those of corresponding haloacids. Explain.
Ans. The amino acids exist as zwitter ions, H3N+ — CHR-COO-. Due to this dipolar salt like character, they have strong dipole-dipole attractions. Therefore, their melting points are higher than corresponding haloacids which do not have salt like character.
Due to salt like character, amino acids intereact strongly with water. As a result, their solubility in water is higher than corresponding haloacids which do not have salt like character.
14.5 Where does the water present in the egg go after boiling the egg?
Ans. When egg is boiled, proteins first undergo denaturation and then coagulation and the water present in the egg gets absorbed in coagulated protein, probably through H- bonding.
14.6 Why cannot Vitamin C be stored in our body?
Ans. Vitamin C cannot be stored in the body because it is water-soluble and is, therefore, easily excreted in urine.
14.7 What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Ans. The products obtained are 2-deoxy-D-ribose, phosphoric acid and thymine.
14.8 When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Ans. A DNA molecule has two strands in which the four complementary bases pair each other, i.e., cytosine (C) always pair with guanine (G) while thymine (T) always pairs with adenine (A). Thus, when a DNA molecule is hydrolysed, the molar amounts of cytosine is always equal to that of guanine and that of adenine is always equal to thymine. In RNA, there is no relationship between the quantities of four bases (C, G, A and U) obtained, therefore, the base pairing principle, i.e. A pairs with U and C pairs with G is not followed. Therefore, unlike DNA, RNA has a single strand.
NCERT EXRECISES
14.1 What are monosaccharides ?
Ans. Monosaccharides are carbohydrates Which cannot be hydrolysed to smaller molecules. Their general formula is (CH2O)n Where n=3-7 These are of two types: Those which contain an aldehyde group (-CHO) are called aldoses and those which contain a keto (C=O) group are called ketoses.
They are further classified as trioses , tetroses ,pentoses , hexoses and heptoses according as they contain 3,4,5,6, and 7 carbon atoms respectively. For example.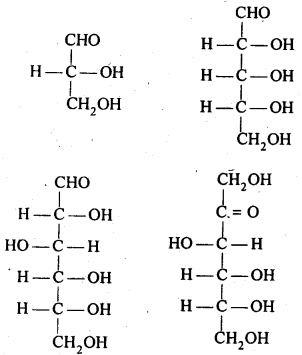
14.2 What are reducing sugars?
Ans. Carbohydrates which reduces Fehling’s solution to red precipitate of Cu2O or Tollen’s reagent to metallic Ag are called reducing sugars. All monosaccharides (both aldoses and ketoses) and disaccharides except sucrose are reducing sugars. Thus, D – (+) – glucose, D-(-)-fructose, D – (+) – maltose and D – (+) – lactose are reducing sugars.
14.3 Write two main functions of carbohydrates in plants.
Ans. Two major functions of carbohydrates in plants are following
(a)Structural material for plant cell walls: The polysaccharide cellulose acts as the chief structural material of the plant cell walls.
(b)Reserve food material: The polysaccharide starch is the major reserve food material in the plants. It is stored in seeds and act as the reserve food material for the tiny plant till it is capable of making its own food by photosynthesis.
14.4 Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Ans. Monosaccharides: Ribose, 2-deoxyribose, galactose and fructose. Disaccharides: Maltose and lactose.
14.5 What do you understand by the term glycosidic linkage?
Ans. The ethereal or oxide linkage through which two monosaccharide units are joined together by the loss of a water molecule to form a molecule of disaccharide is called the glycosidic linkage. The glycosidic linkage in maltose molecule is shown below: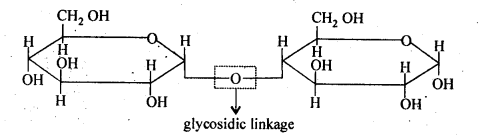
14.6 What is glycogen? How is it different from starch?
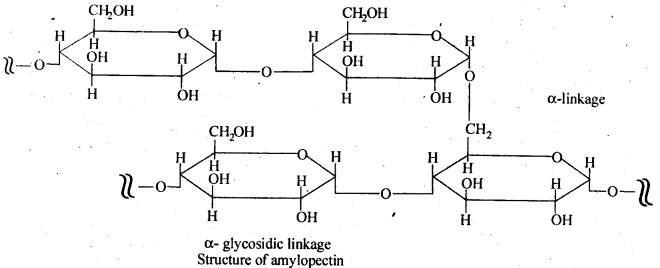
Ans. Glycogen is a condensation polymer of α-D glucose. Starch is not a single compound but is a mixture of two components—a water-soluble component called amylase (15- 20%) and water insoluble component amylopectin (80 – 85%). Amylose is a linear polymer of α – D – glucose. But both glycogen and amylopectin are branched polymers of α – D – glucose; father glycogen is more highly branched than amylopectin as amylopectin chains consists of 20 – 25 glucose units, glycogen chains consist of 10 – 14 glucose units.
14.7 What are the hydrolysis products of (i) sucrose, and (ii) lactose?
Ans. Both sucrose and lactose are disaccharides. Sucrose on hydrolysis gives one molecule each of glucose and fructose but lactose on hydrolysis gives one molecule each of glucose and galactose.
14.8 What is the basic structural difference between starch and cellulose?
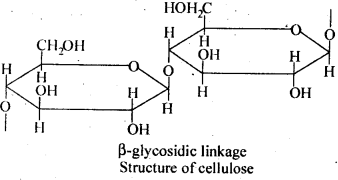
Ans. Starch consists of amylose and amylopectin. Amylose is a linear polymer of α-D-glucose while cellulose is a linear polymer of β -D- glucose. In amylose, C -1 of one glucose unit is connected to C – 4 of the other through α-glycosidic linkage. However, in cellulose, C – 1 of one glucose unit is connected to C-4 of the other through β – glycosidic linkage. Amylopectin on the other hand has highly branched structure.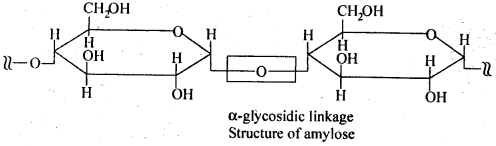
14.9 What happens when D-glucose is treated with . the following reagents.
(i)HI (ii) Bromine water (iii) HNO3
Ans.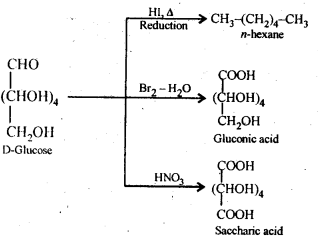
14.10 Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Ans. (a) D (+) – glucose does not undergo certain characteristic reactions of aldehydes, e.g., glucose does not form NaHSO3 addition product.
(b)Glucose reacts with NH2OH to form an oxime but glucose pentaacetate does not. This implies that the aldehydic group is absent in glucose pentaacetate.
(c)D – (+) – glucose exists in two stereoisomeric forms, i.e., α -glucose and β-glucose.
(d)Both α – D – glucose and β – D – glucose undergo mutarotation in aqueous solution. Although the crystalline forms of α- and β -D (+) – glucose are quite stable in aqueous solution but each form slowly changes into an equilibrium mixture of both.
(e)D (+) – glucose forms two isomeric methyl glucosides. Aldehydes normally react with two moles of methanol per mole of the aldehyde to form an acetal but D (+) – glucose when treated with methanol in presence of dry HCl gas, reacts with only one mole of methanol per mole of glucose to form a mixture of two methyl D – glucosides i. e., methyl – α – D – glucoside (melting point 43 8 K, specific rotation +158°) and methyl – β – D – glucoside (melting point 308 K, specific rotation – 33°).
14.11 What are essential and non-essential amino acids? Give two examples of each type.
Ans. α-Amino acids which are needed for good health and proper growth of human beings but are not synthesized by the human body are called- essential amino acids. For example, valine, leucine, phenylalanine, etc. On the other hand, α-amino acids which are needed for health and growth of human beings and are synthesized by the human body are called non-essential amino acids. For example, glycine, alanine, aspartic acid etc.
14.12 Define the following as related to proteins:
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation
Ans. (i) Peptide bond: Proteins are condensation polymers of α-amino acids in which the same or different α-amino acids are joined by peptide bonds. Chemically, a peptide bond is an amide linkage formed between – COOH group of one α-amino acid and -NH-, group of the other α-amino acid by loss of a molecule of water. For example,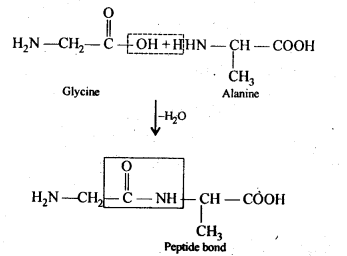
(ii) Primary structure: Proteins may contain one or more polypeptide chains. Each polypeptide chain has a large number of α-amino acids which are linked to one another in a specific manner. The specific sequence in which the various amino acids present in a protein linked to one another is called its primary structure. Any change in the sequence of α-amino acids creates a different protein.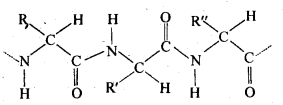
(iii) Denaturation: Each protein in the biological system has a unique three-dimensional structure and has specific biological activity. This is called native form of a protein. When a protein in its native form is subjected to a physical change such as change in temperature or a chemical change like change in pH, etc., hydrogen bonds gets broken. As a result, soluble forms of proteins such as globular proteins undergo coagulation or precipitation to give fibrous proteins which are insoluble in water. This coagulation also results in loss of biological activity of the proteins and this loss in biological activity, is called denaturation
One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
Chapter End Question
13. What are the common types of secondary structure of proteins?
Ans. There are two common types of secondary structure of proteins:
- -helix structure
- pleated sheet structure
- Helix structure: In this structure, the -NH group of an amino acid residue forms H-bond with the  group of the adjacent turn of the right-handed screw (
group of the adjacent turn of the right-handed screw ( -helix).
-helix).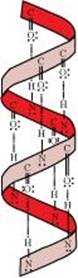
pleated sheet structure: This structure is called so because it looks like the pleated folds of drapery. In this structure, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. These peptide chains are held together by inter-molecular hydrogen bonds.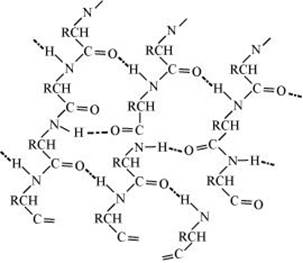
14. What type of bonding helps in stabilising the -helix structure of proteins?
Ans. The H-bonds formed between the -NH group of each amino acid residue and the 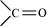 group of the adjacent turns of the -helix help in stabilising the helix.
group of the adjacent turns of the -helix help in stabilising the helix.
15. Differentiate between globular and fibrous proteins.
Ans.
| Fibrous protein | Globular protein |
| It is a fibre-like structure formed by the polypeptide chain. These proteins are held by strong hydrogen and disulphide bonds. | The polypeptide chain in this protein is folded around itself, giving rise to a spherical structure. |
| It is usually insoluble in water | It is usually soluble in water |
| Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair, collagen in tendons; and myosin in muscles. | All enzymes are globular proteins. Some hormones such as insulin are also globular proteins. |
16. How do you explain the amphoteric behavior of amino acids?
Ans. In aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to give a dipolar ion known as zwitter ion.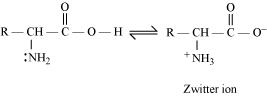
Therefore, in zwitter ionic form, the amino acid can act both as an acid and as a base.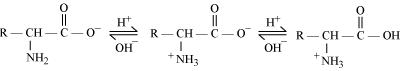
Thus, amino acids show amphoteric behaviour.
17. What are enzymes?
Ans. Enzymes are proteins that catalyse biological reactions. They are very specific in nature and catalyse only a particular reaction for a particular substrate. Enzymes are usually named after the particular substrate or class of substrate and sometimes after the particular reaction.
For example, the enzyme used to catalyse the hydrolysis of maltose into glucose is named as maltase.
Again, the enzymes used to catalyse the oxidation of one substrate with the simultaneous reduction of another substrate are named as oxidoreductase enzymes.
The name of an enzyme ends with '-ase'.
18. What is the effect of denaturation on the structure of proteins?
Ans. As a result of denaturation, globules get unfolded and helixes get uncoiled. Secondary and tertiary structures of protein are destroyed, but the primary structures remain unaltered. It can be said that during denaturation, secondary and tertiary-structured proteins get converted into primary-structured proteins. Also, as the secondary and tertiary structures of a protein are destroyed, the enzyme loses its activity.
19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Ans. On the basis of their solubility in water or fat, vitamins are classified into two groups.
(i) Fat-soluble vitamins: Vitamins that are soluble in fat and oils, but not in water, belong to this group. For example: Vitamins A, D, E, and K
(ii) Water-soluble vitamins: Vitamins that are soluble in water belong to this group. For example: B group vitamins (B1, B2, B6, B12, etc.) and vitamin C
However, biotin or vitamin H is neither soluble in water nor in fat.
Vitamin K is responsible for the coagulation of blood.
20. Why are vitamin A and vitamin C essential to us? Give their important sources.
Ans. The deficiency of vitamin A leads to xerophthalmia (hardening of the cornea of the eye) and night blindness. The deficiency of vitamin C leads to scurvy (bleeding gums).
The sources of vitamin A are fish liver oil, carrots, butter, and milk. The sources of vitamin C are citrus fruits, amla, and green leafy vegetables.
21. What are nucleic acids? Mention their two important functions.
Ans. Nucleic acids are biomolecules found in the nuclei of all living cells, as one of the constituents of chromosomes. There are mainly two types of nucleic acids - deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are also known as polynucleotides as they are long-chain polymers of nucleotides.
Two main functions of nucleic acids are:
(i) DNA is responsible for the transmission of inherent characters from one generation to the next. This process of transmission is called heredity.
(ii) Nucleic acids (both DNA and RNA) are responsible for protein synthesis in a cell. Even though the proteins are actually synthesised by the various RNA molecules in a cell, the message for the synthesis of a particular protein is present in DNA.
22. What is the difference between a nucleoside and a nucleotide?
Ans. A nucleoside is formed by the attachment of a base to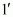 position of sugar.
position of sugar.
Nucleoside = Sugar + Base
On the other hand, all the three basic components of nucleic acids (i.e., pentose sugar, phosphoric acid, and base) are present in a nucleotide.
Nucleotide = Sugar + Base + Phosphoric acid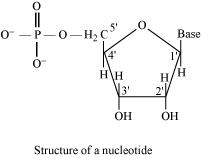
23. The two strands in DNA are not identical but are complementary. Explain.
Ans. In the helical structure of DNA, the two strands are held together by hydrogen bonds between specific pairs of bases. Cytosine forms hydrogen bond with guanine, while adenine forms hydrogen bond with thymine. As a result, the two strands are complementary to each other.
24. Write the important structural and functional differences between DNA and RNA.
Ans. The structural differences between DNA and RNA are as follows:
| DNA | RNA |
| The sugar moiety in DNA molecules is -D-2 deoxyribose. | The sugar moiety in DNA molecules is -D-deoxyribose. |
| DNA contains thymine(T). It does not contain uracil(U). | RNA contains uracil(U). It does not contain thymine(T). |
| The helical structure of DNA is double-stranded. | The helical structure of RNA is single-stranded. |
The functional differences between DNA and RNA are as follows:
| DNA | RNA |
| DNA is the chemical basis of heredity. | RNA is not responsible for heredity. |
| DNA molecules do not synthesise proteins, but transfer coded message for the synthesis of proteins in the cells. | Proteins are synthesised by RNA molecules in the cells. |
25. What are the different types of RNA found in the cell?
Ans. (i) Messenger RNA (m-RNA)
(ii) Ribosomal RNA (r-RNA)
(iii) Transfer RNA (t-RNA)