Biomolecules-Test Papers
CBSE Test Paper-01
Class - 12 Chemistry (Biomolecules)
- ____ are joined together by phosphodiester linkage between 5′ and 3′ carbon atoms of the pentose sugar.
- Nucleosides
- Nucleic acids
- Proteins
- Nucleotides
- The following molecule is called as
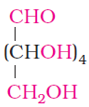
- carbohydrate
- Vitamin
- Protein
- Lipid
- One or more of the following vitamin is insoluble in water
- all of these
- vitamin D
- vitamin K
- Vitamin E
- Night blindness is caused by the deficiency of
- Vitamin D
- Vitamin B
- Vitamin C
- Vitamin A
- Oxime is formed by treating glucose with
- Water
- nitric acid
- bromine water
- hydroxylamine
Name the forces responsible for secondary and tertiary structure.
Write name of linkage joining two amino acids.
Deficiency of which vitamin causes scurvy?
How do epimers differ from anomers?
What type of bonding helps in stabilising the -helix structure of proteins?
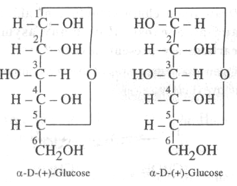
Distinguish between -glucose and -glucose.
- Define the terms as related to proteins:
- Peptide linkage
- Primary structure
- Denaturation
What are nucleotides? Name two classes of nitrogen containing bases found amongst nucleotides.
What are the common types of secondary structure of proteins?
- Define the following terms:
- Co-enzymes
- Mutation in biomolecules
- List four main functions of carbohydrate in organism.
CBSE Test Paper-01
Class - 12 Chemistry (Biomolecules)
Solutions
- (d) Nucleotides
Explanation: When nucleoside is linked to phosphoric acid at 5′-position of sugar moiety, we get a nucleotide and nucleotides are joined by a phosphodiester bond between 5′ and 3′ carbon atoms of the pentose sugar. - (a) carbohydrate
Explanation: It is aldohexose. An aldohexose is a hexose with an aldehyde group on one end.it is naturally occurring in nature and is found in fruits. - (a) all of these
Explanation: Vitamins which are soluble in fat and oils but insoluble in water are kept in fat soluble vitamins. These are vitamins A, D, E and K. They are stored in liver and adipose (fat storing) tissues. All these are insoluble in water. - (d) Vitamin A
Explanation: Vitamin A keeps eyes in good health. Deficiency of Vitamin A causes Night Blindness. - (d) hydroxylamine
Explanation: Glucose reacts with hydroxylamine (NH2OH) to form an oxime. Oxime is formed by combination of carbonyl group of glucose and hydroxyl group of hydroxyl amine. - The forces which are responsible for tertiary structure of proteins are hydrogen bonds, disulphide linkage, vanderwalls and electrostatic forces of attraction.
- Peptide linkage
- Vitamin C
- Carbohydrate which differ in configuration at the glycosidic carbon (i.e., C1 in aldoses and C2 in ketoses) are called anomers while those which differ in configuration at any carbon other than glycosidic carbon are called epimers. For example, -D - glucose and - D- glucose are anomers since they differ in configuration at C2 (other than the glycosidic carbon C1. In other words, glucose and monose are C2 epimers, similarly, we can show that glucose and galactose are C2 epimers since they differ in configuration only at C4.
- The H-bonds formed between the -NH group of each amino acid residue and the
 group of the adjacent turns of the -helix help in stabilising the helix.
group of the adjacent turns of the -helix help in stabilising the helix. - -glucose and -glucose differ in configuration at C-1. They Differ in optical rotation. The differ in melting point also. Such isomers differing in configuration at C-1 are called anomers.
- Peptide linkage:
The amide formed between -COOH group of one molecule of an amino acid and group of another molecule of the amino acid by the elimination of a water molecule is called a peptide linkage.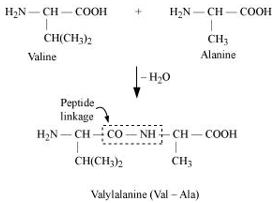
- Primary structure: The primary structure of protein refers to the specific sequence in which various amino acids are present in it, i.e., the sequence of linkages between amino acids in a polypeptide chain. The sequence in which amino acids are arranged is different in each protein. A change in the sequence creates a different protein.
- Denaturation: In a biological system, a protein is found to have a unique 3-dimensional structure and a unique biological activity. In such a situation, the protein is called native protein. However, when the native protein is subjected to physical changes such as change in temperature or chemical changes such as change in pH, its H-bonds are disturbed. This disturbance unfolds the globules and uncoils the helix. As a result, the protein loses its biological activity. This loss of biological activity by the protein is called denaturation. During denaturation, the secondary and the tertiary structures of the protein get destroyed, but the primary structure remains unaltered.
One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
- Peptide linkage:
- Nucleotides are monomer of nucleic acid. They are made up of heterocyclic base containing nitrogen, a fine carbon sugar and a phosphate group etc. e.g. AMP, ADP. They consists of heterocyclic bases. Adenine (A), Guanine (G), Cytosine (C) Thymine (T) and uracil (u).
- There are two common types of secondary structure of proteins: - Helix structure
In this structure, the -NH group of an amino acid residue forms H-bond with the group of the adjacent turn of the right-handed screw ( -helix).
group of the adjacent turn of the right-handed screw ( -helix).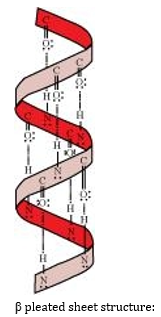
pleated sheet structure:
This structure is called so because it looks like the pleated folds of drapery. In this structure, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. These peptide chains are held together by intermolecular hydrogen bonds.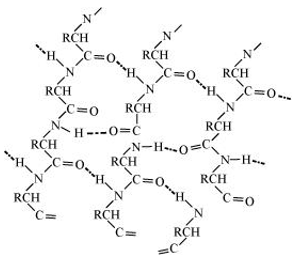
- -helix structure
- pleated sheet structure
- Co-enzymes: Prosthetic groups which get attached to enzymes at the time of reaction are called co-enzyme. They increase the activity of enzymes.
- Mutation in biomolecules: A Difference of a single base in DNA molecule can cause change in amino acid sequence which leads to mutation.
- Nucleotides: They are monomers of nucleic acids. They consist of heterocylic base. Pentose sugar and phosphoric acid residues.
Function of carbohydrate:- They act as biofuel.
- Cellulose forms cell wall of the plants.