Haloalkanes and Haloarenes-Revision Notes
CBSE Class 12 Chemistry
Quick Revision Notes
Chapter 10
Haloalkanes and Haloarenes
- Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having a partial positive charge and X having a partial negative charge.
- Preparation of haloalkanes:
a)
b)
c)
d) Halogen Exchange Method:
- Preparation of haloarenes:
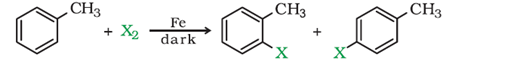
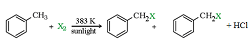
a) By elecrophilic substitution reaction:
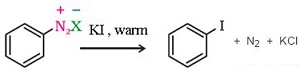
b) Sandmeyer’s reaction: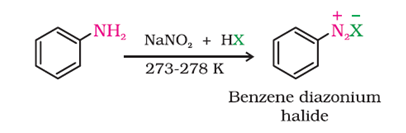
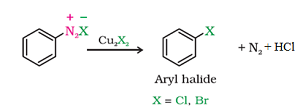
c) Gattermann reaction: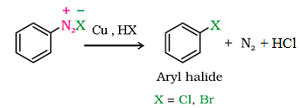
d) From Diazonium Chloride:
e). Balz – Schiemann reaction: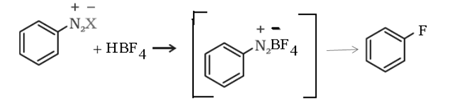
- Physical properties of haloalkanes:
a) Solubility
- Although haloalkanes are polar in nature, yet they are practically very slightly soluble in water.
- In order for a haloalkane to dissolve in water, energy is required to overcome the attractions between the haloalkane molecules and break the hydrogen bonds between water molecules.
- However Haloalkanes are not able to form hydrogen bonds with water and therefore, less energy is released when new attractions are set up between the haloalkane and the water molecules because these are not as strong as the original hydrogen bonds in water molecules.
- As a result, solubility of haloalkanes in water is low.
b) Density
- Simple fluoro and chloroalkanes are lighter than water while bromides and polychlorodevrivatives are heavier than water.
- With the increase in number of carbon atoms, the densities go on increasing. With the increase in number of halogen atoms, the densities go on increasing. The densities increase in the order: Fluoride < chloride < bromide < iodide
- The density also increases with increasing number and atomic mass of the halogen.
c) Boiling Points
- Molecules of organic halogen compounds are generally polar.
- Due to the polarity as well as higher molecular mass as compared to the parent hydrocarbon, the intermolecular forces of attraction (dipole – dipole and van der Waals) between the molecules are stronger in halogen derivatives of alkanes.
- As a result melting and boiling points of chlorides, bromides and iodides are considerably higher than those of the parent hydrocarbon of comparable molecular mass.
- For the same alkyl group the boiling points of alkyl chlorides, bromides and iodides follow the order RI >RBr>RCl> RF where R is an alkyl group. This is because with the increase in the size of the halogen, the magnitude of van der Waals force increase.
- In general, the boiling points of chloro, bromo and iodo compounds increase with increase in the number of halogen atoms.
- For the same halogen atom, the boiling points of haloalkanes increase with increase in the size of alkyl groups.
- For isomeric alkyl halides, the boiling points decrease with branching. This is because branching of the chain makes the molecule more compact and, therefore, decrease the surface area. Due to decrease in surface area, the magnitude of van der Waals forces of attraction decreases and consequently, the boiling points of the branched chain compound is less than those of the straight chain compounds.
- Physical Properties of Haloarenes:
a. These are generally colourless liquids or crystalline solids.
b. These are heavier than water.
c. Melting and boiling points of haloarenes
i. Melting and boiling points of haloarenes are nearly the same as those of alkyl halides containing the same number of carbon atoms.
ii. The boiling points of monohalogen derivatives of benzene are in the order: iodo>bromo>chloro>fluoro
iii. For the same halogen atom, the melting and boiling points increase as the size of the aryl group increases.
iv. The melting point of para isomer is quite higher than that of ortho or meta isomers. This is due to the fast that is has symmetrical structure and therefore, its molecules can easily pack loosely in the crystal lattice. As a result intermolecular forces of attraction are stronger and therefore, greater energy is required to break its lattice and it melts at higher temperature.
- Chemical properties of haloalkanes:
Nucleophilic substitution reaction:
Mechanism of Nucleophilic Substitution Reaction:
SN1 Mechanism
- First order reaction.
- Rate = k [RX] [Nu]
- Racemic mixture
- One step reaction
- Order: CH3X < 10< 20< 30
SN2 Mechanism
- Second order reaction
- Rate = k [RX]
- Inversion of configuration
- Two step reaction
- Order: CH3X > 10> 20> 30
- Elimination reaction: Dehydrohalogentaion(- elimination): When a haloalkane with β-hydrogen atom is heated with alcoholic solution of potassium hydroxide, there is elimination of hydrogen atom from β-carbon and a halogen atom from the α-carbon atom. As a result, an alkene is formed as a product. Zaitsev rule (also pronounced as Saytzeff) is followed.It states that “In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
- Reaction with metals:
a) Reaction with Magnesium
b) Wurtz reaction
- Chemical properties of haloarenes:
a) Dow’s Process
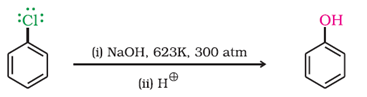
b) With halogens
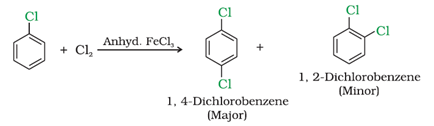
c) With conc. nitric and sulphuric acid
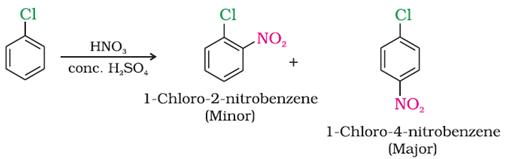
d) On heating with conc. sulphuric acid
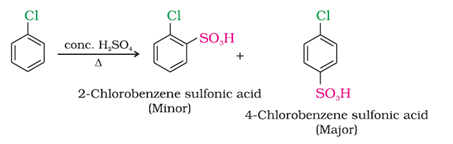
e) With methyl chloride
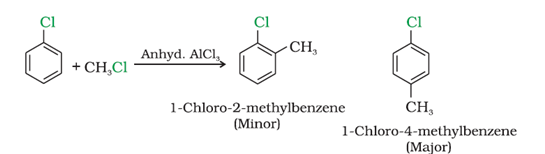
f) With acetyl chloride
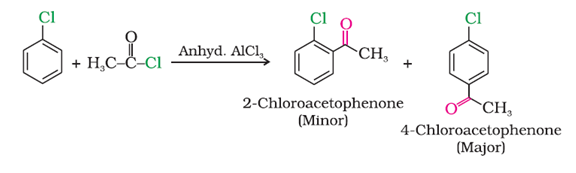
g) Fittig reaction:
h) Wurtz – fittig reaction:
i) Other conversions: